T V Diagramm Thermodynamik
Pv nrt consider the p v diagram below in which the system evolves from a b c.
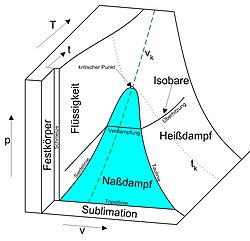
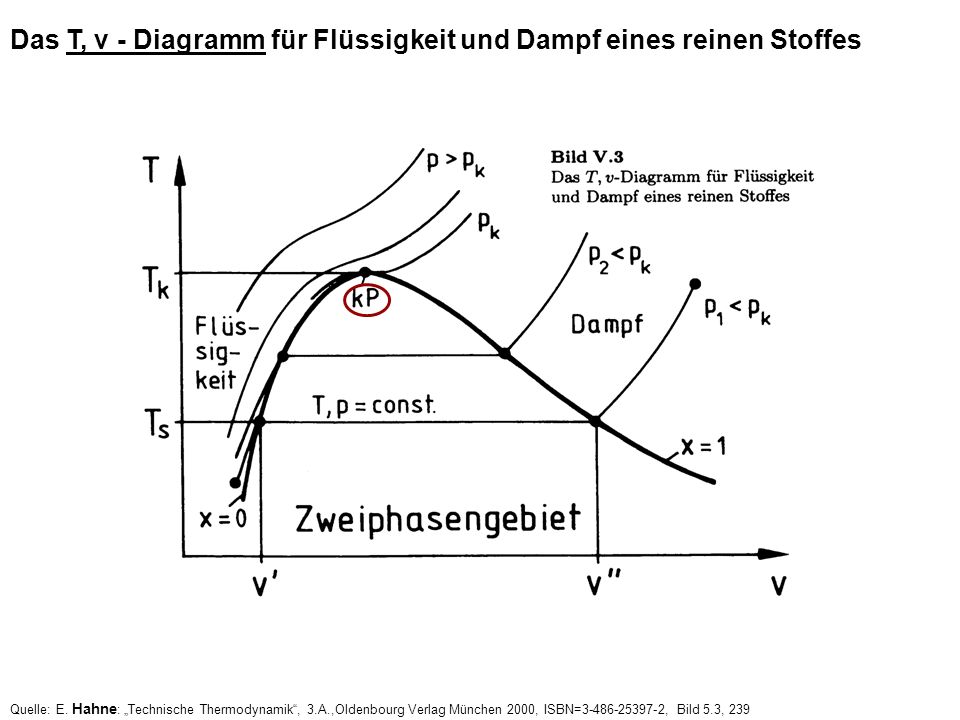
T v diagramm thermodynamik. Water boils at a temperature of 100 0 c. Today we will see here the t v diagram of a pure substance with the help of this post. E change in internal energy.
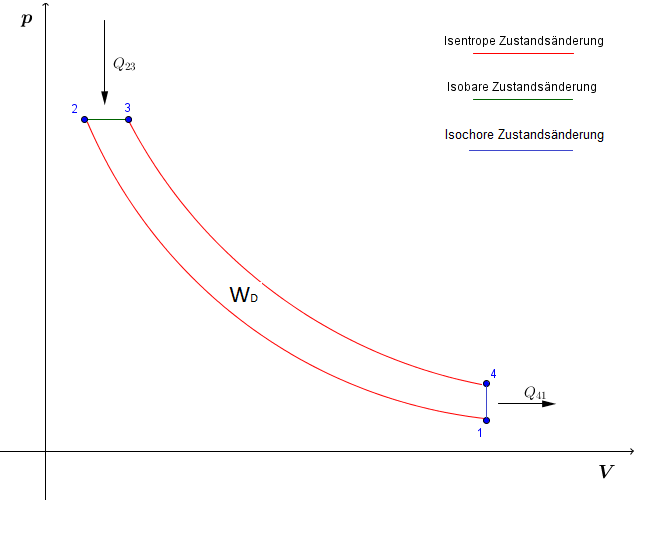
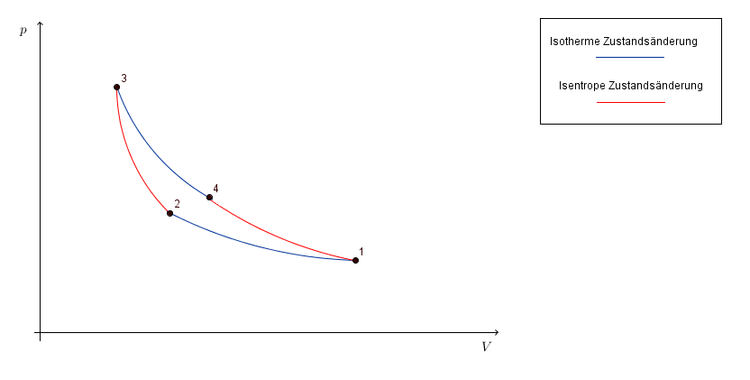
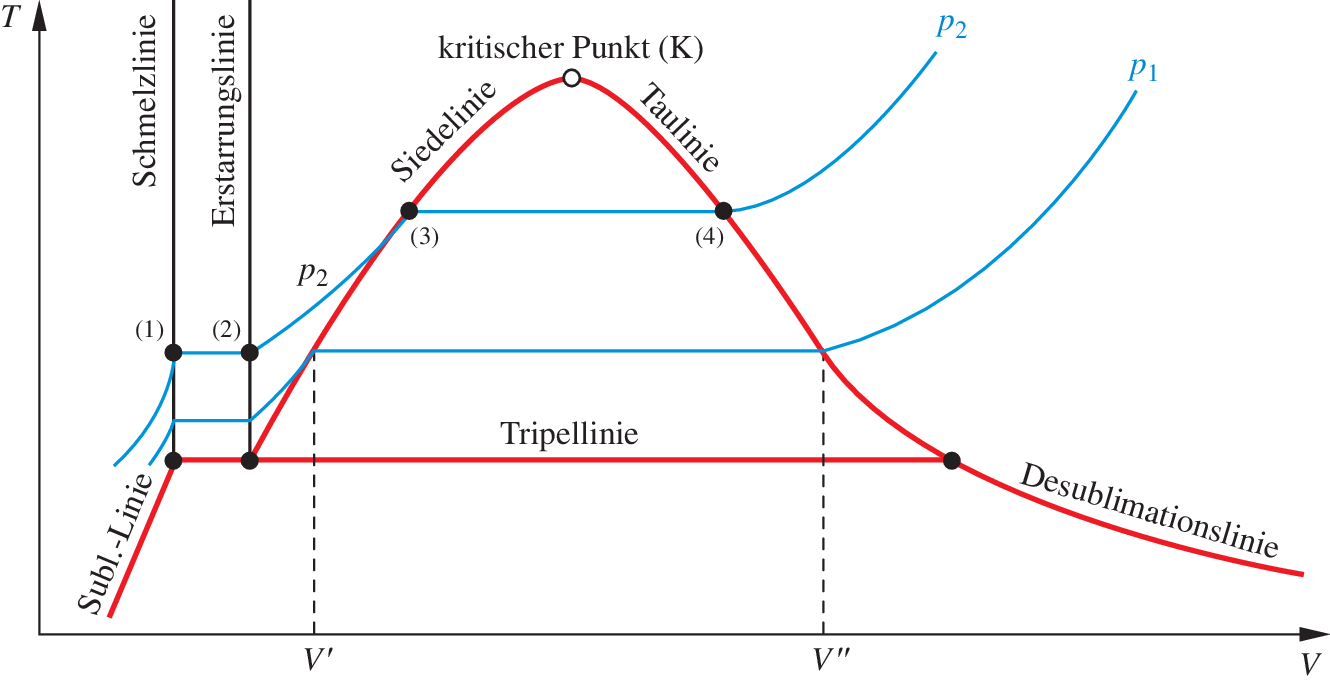
Notice that the t v diagram is based exclusively on intensive properties hence mass is not indicated on the diagram. No this is not correct answer because we have not mentioned the value of pressure here. The isentropic process on p v diagram is represented as shown in fig.
Let us first see here some basic introduction parts and then we will draw the t v diagram of a pure substance. For instance a temperature entropy diagram t s diagram may be used to demonstrate the behavior of a fluid as it is changed by a compressor. Change in internal energy u 2 u 1 m x c v t 2 t 1.
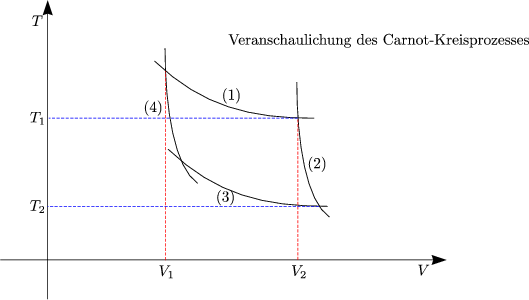
In der thermodynamik sind das p v und t s diagramm wichtige elemente um zustandsänderungen darstellen zu können. Eine zustandsänderung in der thermodynamik beschreibt einen komplexen zusammenhang der drei großen zustandsgrößen druck temperatur und volumen. Another way of determination of change in internal energy is very common in isentropic operation.
Thermodynamic diagrams are diagrams used to represent the thermodynamic states of a material typically fluid and the consequences of manipulating this material. To like social share and comment at the end of the page. The t ɸ diagram shows the entropy remains constant.
This video explains how to draw t v diagrams and what they represent. Question 1 a cyclic process abca as shown in below v t diagram is performed with a constant mass of ideal gas show the process in the p v digram. Das p v und t s diagramm sind spezielle formen von phasendiagrammen wir verwenden es um prozessabläufe zu veranschaulichen wie zum beispiel den otto prozess.
What is the net work done by the system on its environment w. Wir schauen uns in diesem beitrag an was wir anhand dieser ablesen können. C a p nm 2 105 1 t 0 v m3 b 5 104 a 0 j b 5 0 104 j c about 7 0 104 j d 105 j e not enough information to tell 2.
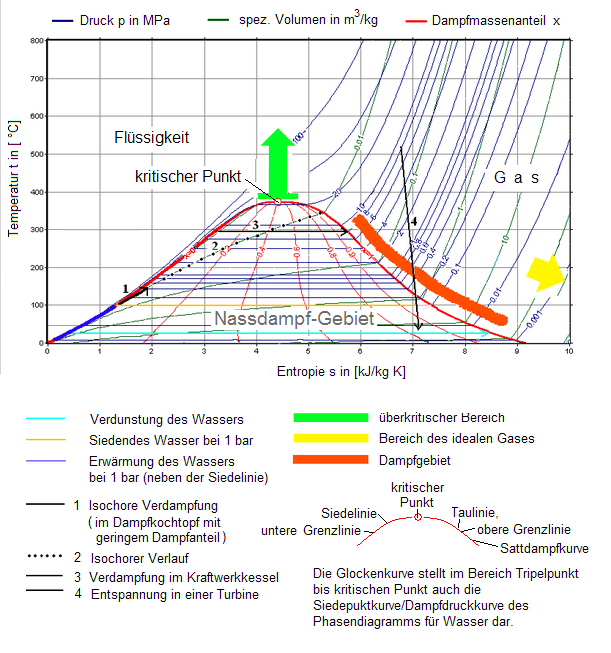
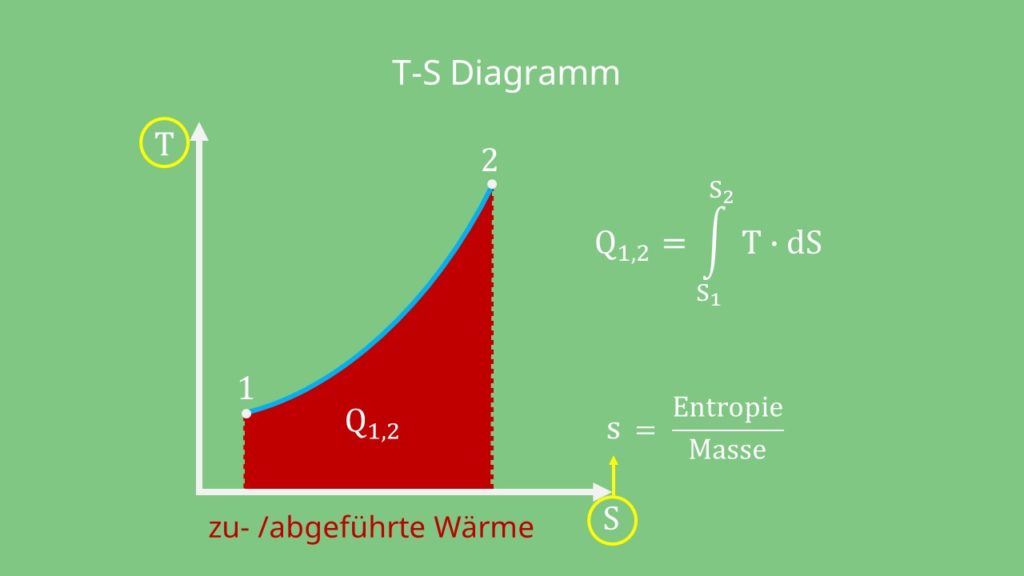
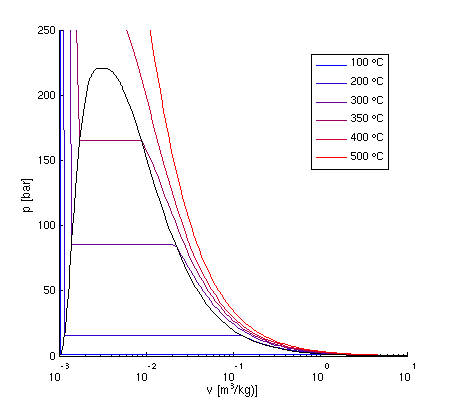
For reversible ideal processes the area under the t s. Thus we indicate on the diagram that in order to determine the quality at state 2 we need to first evaluate the specific volume v 2 which can then be compared to the saturation values v f and v g at the pressure of 100 kpa. V δt ideal gas law.
As usual we get.