P V T Diagram For Water
As described on the work slide the area under a process curve on a p v diagram is equal to the work performed by a gas during the process.
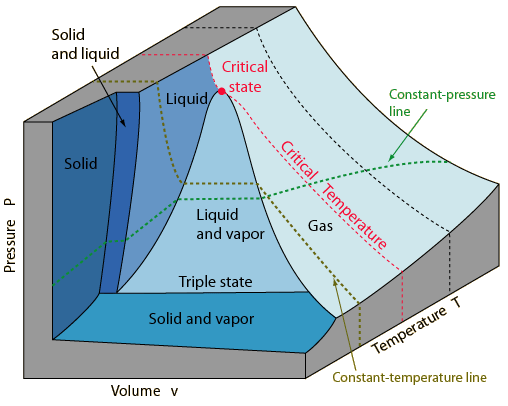
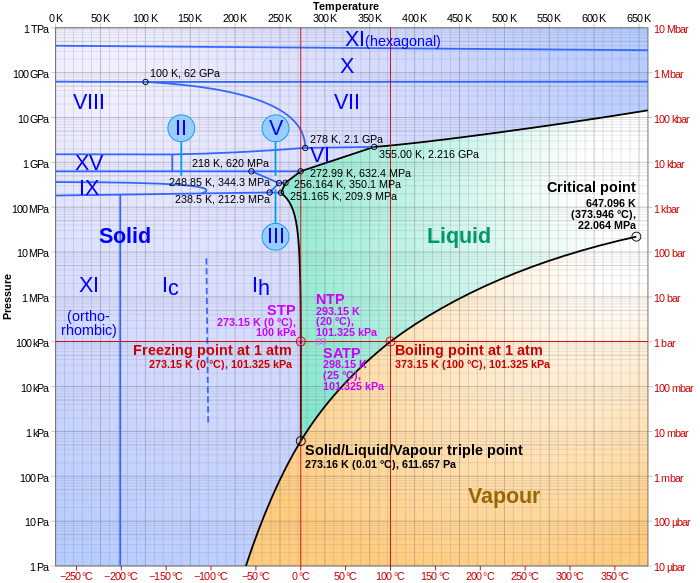
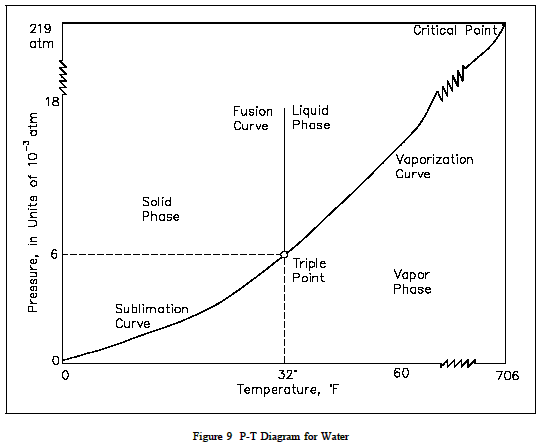
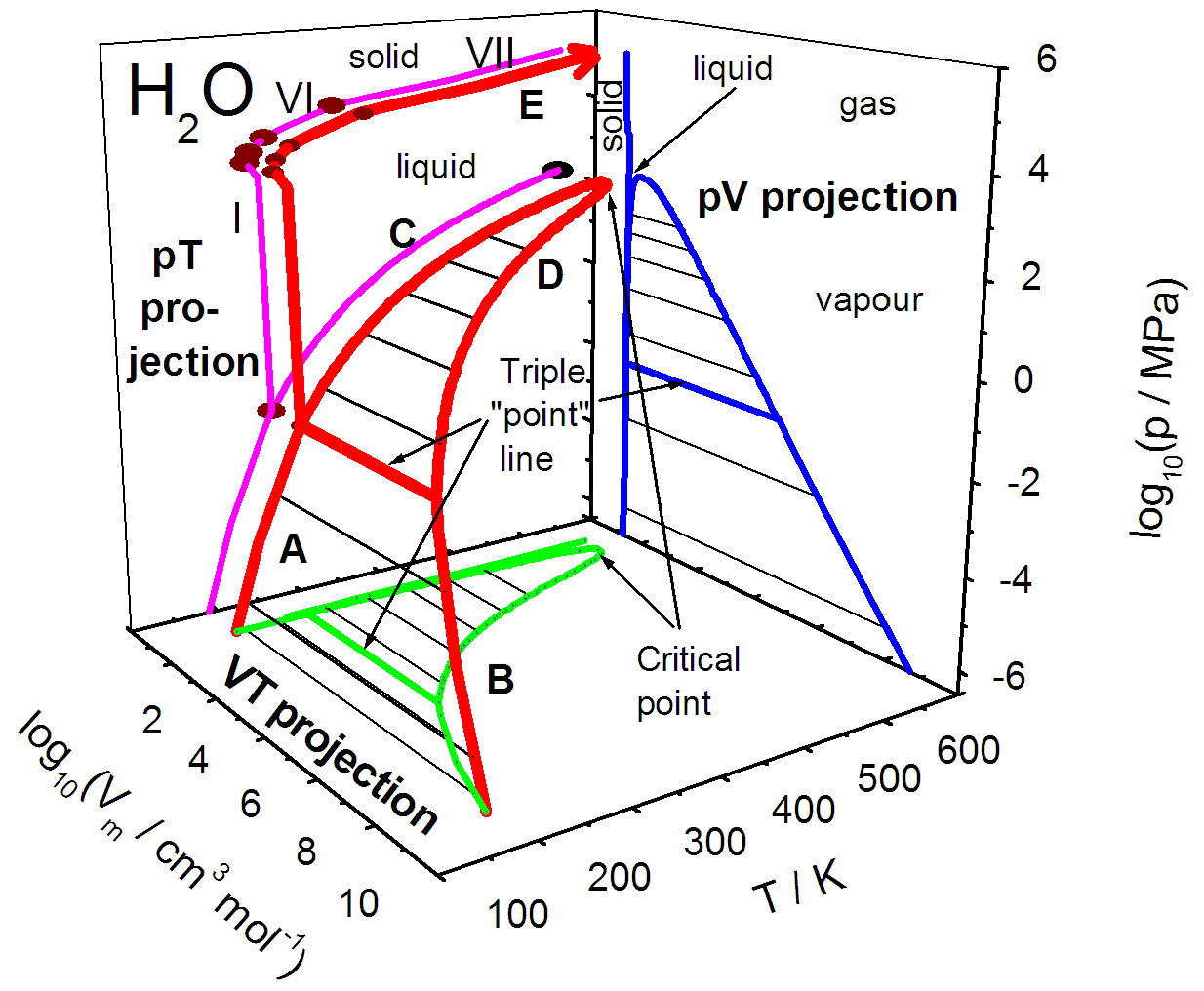
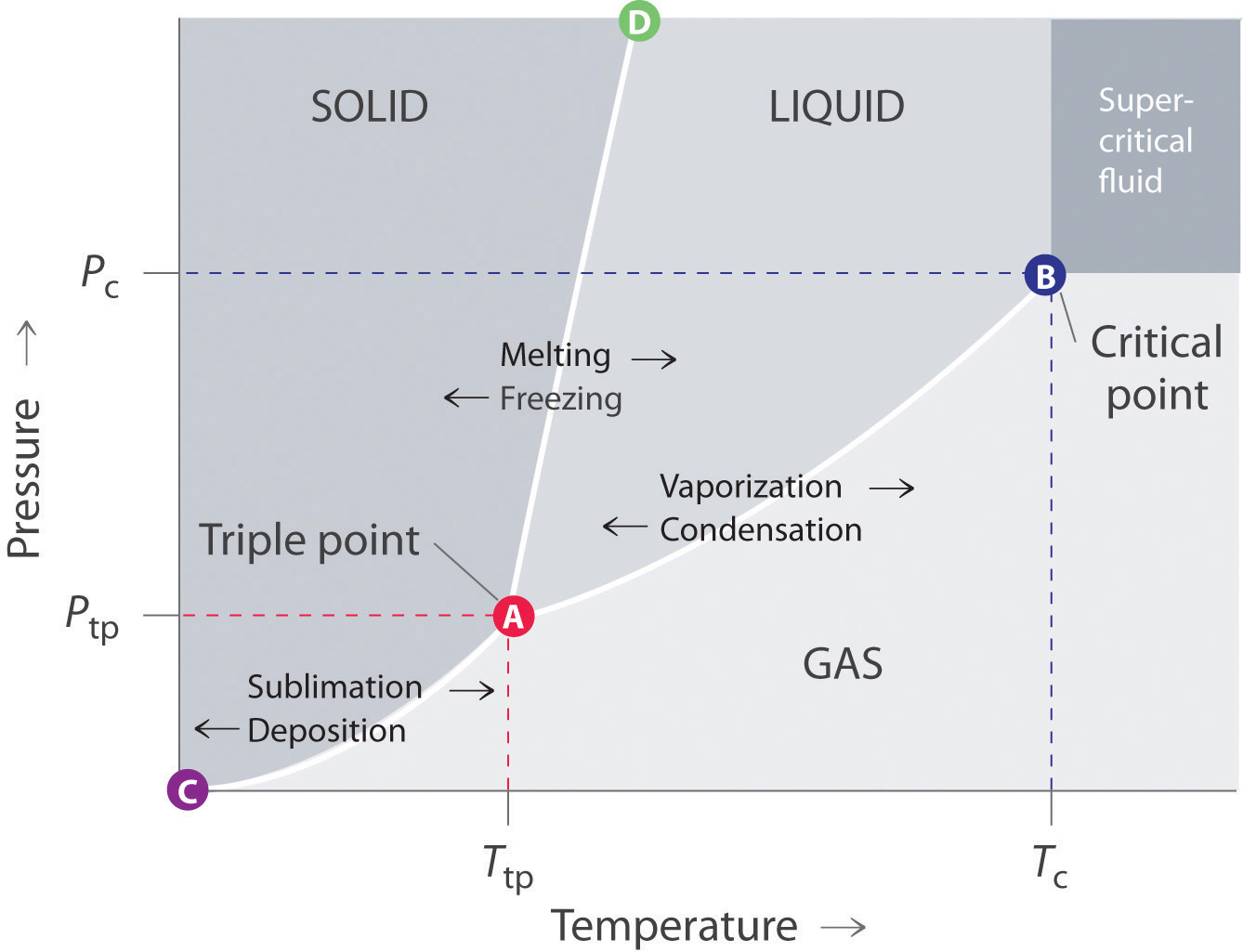
P v t diagram for water. The equilibrium conditions are shown as curves on a curved. Figure 3 1 shows the p v t surface of a substance such as water that expands on freezing. 3d phase diagram of water fluids and selected ices an orthographic projection of the 3d p v t graph showing pressure and temperature as the vertical and horizontal axes collapses the 3d plot into the standard 2d pressure temperature diagram.
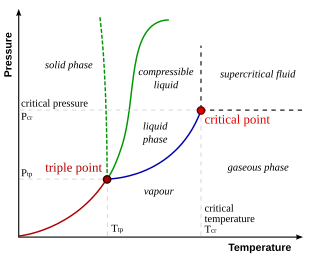
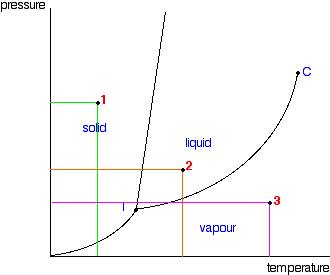
P v diagram for water solid liquid vapor region if we heat ice at different vapor pressures and note down the corresponding change in volumes the saturation state points for solid liquid and vapor state from which a change of phase may occur without change of pressure and temperature for different pressures may be obtained on a p v diagram. Explain why this happens and refer your explanation to the p v t diagram for water shown above. Lines of constant pressure curve from the lower left to upper right on a t s diagram.
When the pressure gets too high for the pipe to contain it ruptures. By considering the piston cylinder device again the temperature keeps constant by heat transfer. It is possible to envision three dimensional 3d graphs showing three thermodynamic quantities.
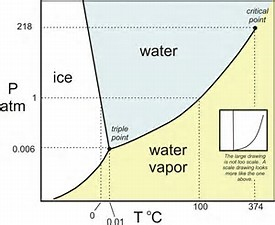
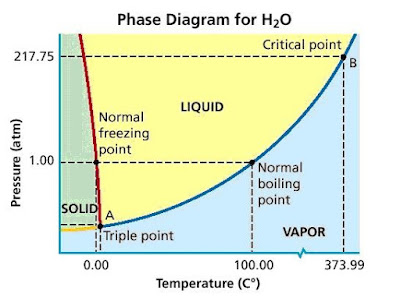
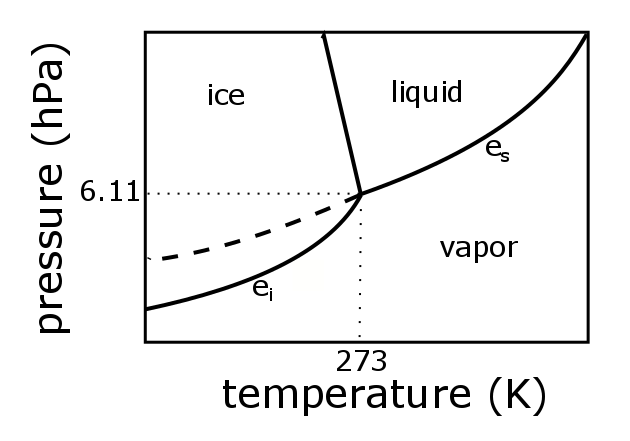
Explore thousands of free applications across science mathematics engineering technology business art finance social sciences and more. Up to 99 63 c the boiling point of water at 0 1 mpa at this pressure water exists as a liquid. Such a 3d graph is sometimes called a p v t diagram.
A 3 d view b p t diagram c p v diagram2. Note that the boiling point of 100 0 c is at a pressure of 0 101325 mpa 1 atm which is the average atmospheric pressure. For example for a single component a 3d cartesian coordinate type graph can show temperature t on one axis pressure p on a second axis and specific volume v on a third.
This plot is called a t s diagram. Water pipes burst because the water inside them expands is it gets close to freezing and this causes an increase in pressure inside the pipe. Figure 3 1 1 p v t surface and projections for a substance that expands on freezing.
Above that it exists as water vapor. The water vaporization process can also be described in p v diagram. Enjoy the videos and music you love upload original content and share it all with friends family and the world on youtube.
The location of a point on the p v t surface gives the values of pressure specific volume and temperature at equilibrium.