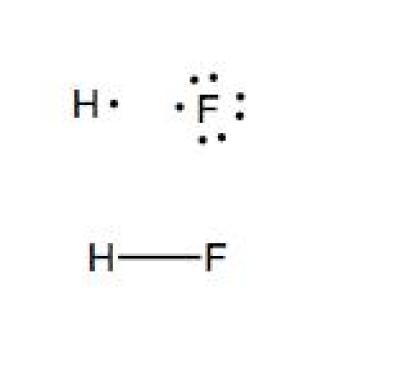Hf Structure Lewis
It is an intermediate for many chemical reactions and syntheses.
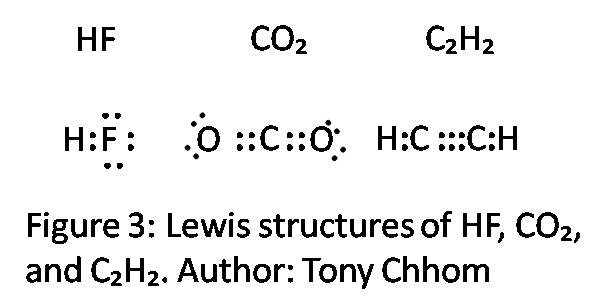
Hf structure lewis. Hydrofluoric acid is a solution of hydrogen fluoride hf in water solutions of hf are colourless acidic and highly corrosive it is used to make most fluorine containing compounds. Hydrogen has 1 valence electron and fluorine in group 7 with f and cl has 7 valence electrons. Hydrogen fluoride is a chemical compound with the chemical formula h f this colorless gas or liquid is the principal industrial source of fluorine often as an aqueous solution called hydrofluoric acid it is an important feedstock in the preparation of many important compounds including pharmaceuticals and polymers e g.
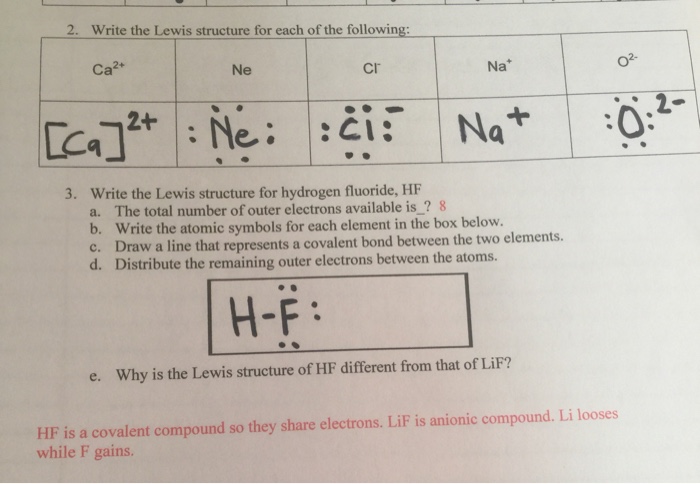
With the lewis structure for hf remember that hydrogen only needs 2 valence electrons to have a full outer shell. I quickly take you through how to draw the lewis structure of hf hydrogen fluoride. Hydrogen fluoride mixes readily with water forming hydrofluoric acid.
Hf was successfully immobilized at the surface of the acf by forming polyfluoride structures. Drawing lewis structures sum the valence electrons from all atoms in the species. The lewis and brønsted acidity are strongly reduced presumably due to the interactions with surface lewis sites as well as with surface fluorides.
Examples include the commonly used pharmaceutical antidepressant medication fluoxetine prozac and the material ptfe teflon. How the molecule might react with other molecules. A lewis structure is a graphic representation of the electron distribution around atoms.
The key is to understand the steps and practice. Hydrogen fluoride hydrofluoric acid is used extensively in the extraction processing and refining of metals rock brick and oil. Teflon hf is widely used in the petrochemical industry as a component of.
For lif we have an ionic compound and we need to take that into account when we draw t. For all practical purposes they are considered the same chemical. A lewis structure also helps to make a prediction about the geometry of a molecule.
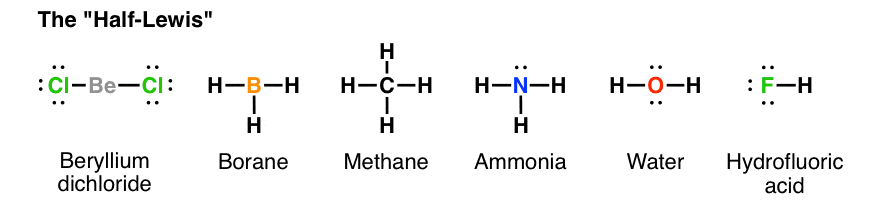
Write the atomic symbols for the atoms involved so as to show which atoms are connected to which. Hf is very similar to hf and hcl. Every chemistry student has to learn how to draw lewis dot structures.
The physical properties of the molecule like boiling point surface tension etc. Elemental fluorine is produced from it. The reason for learning to draw lewis structures is to predict the number and type of bonds that may be formed around an atom.
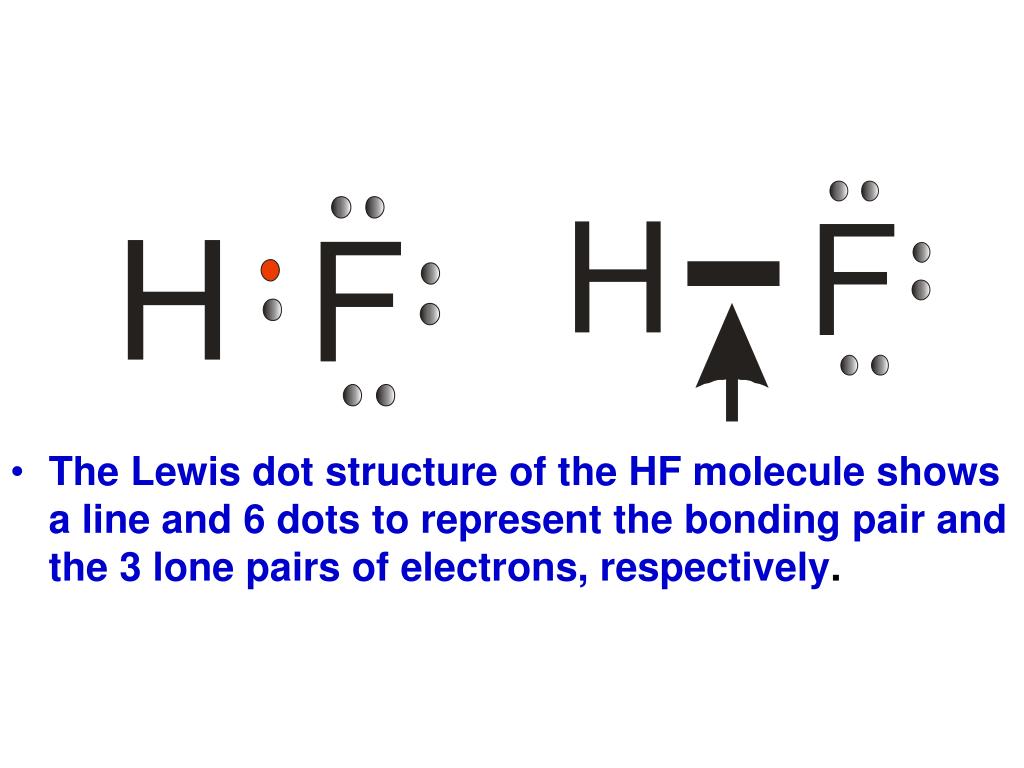
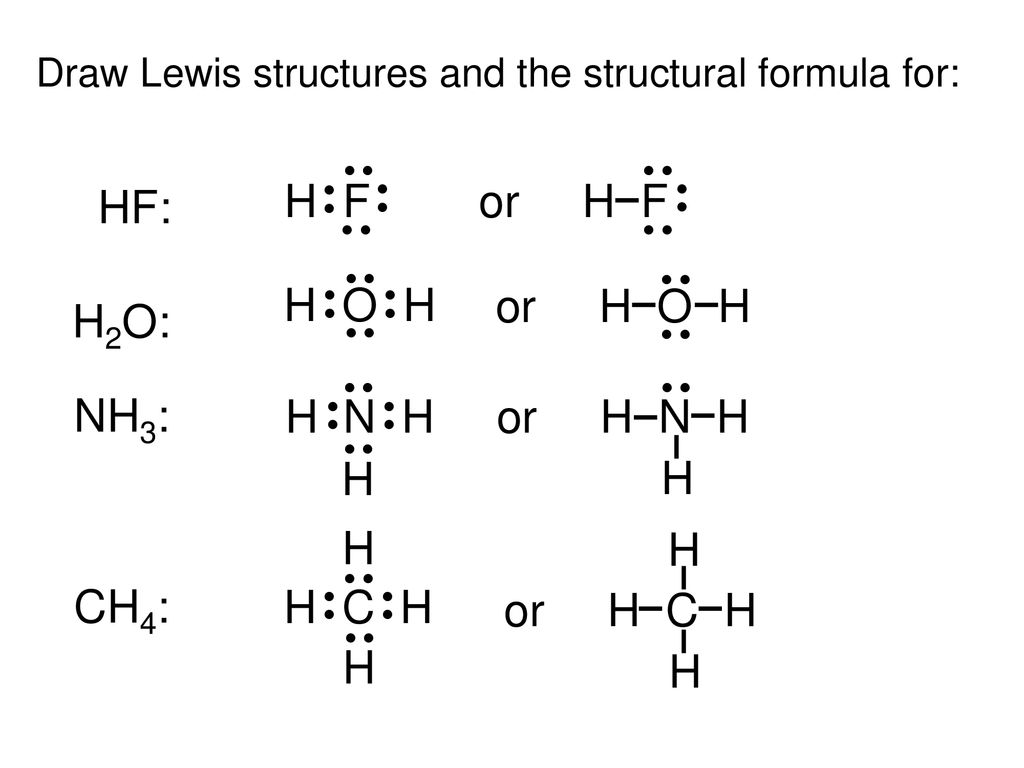
The shape of a molecule. A step by step explanation of how to draw the lif lewis dot structure. Bonding molecular structure page 44 lewis dot structure of hydrogen fluoride.