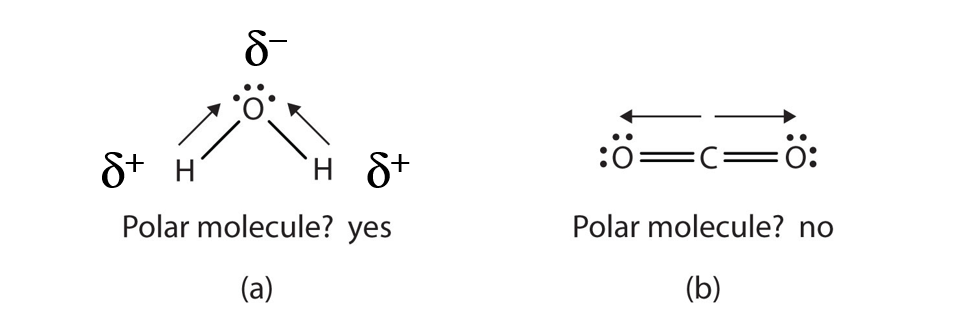Hf Shape And Polarity
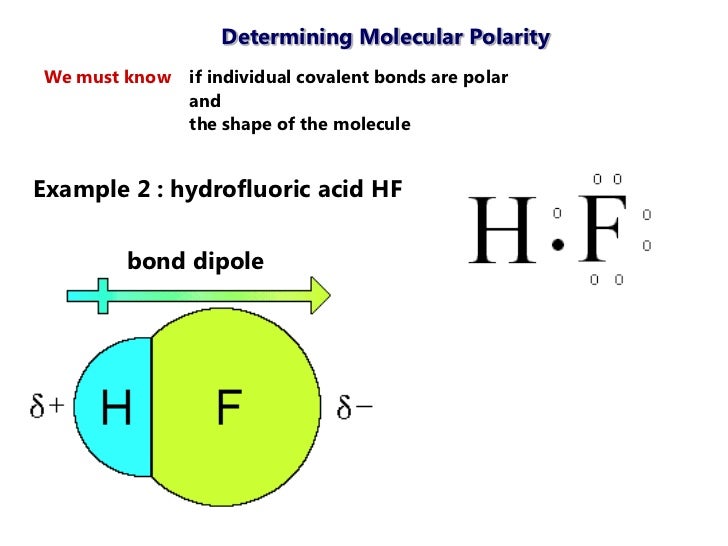
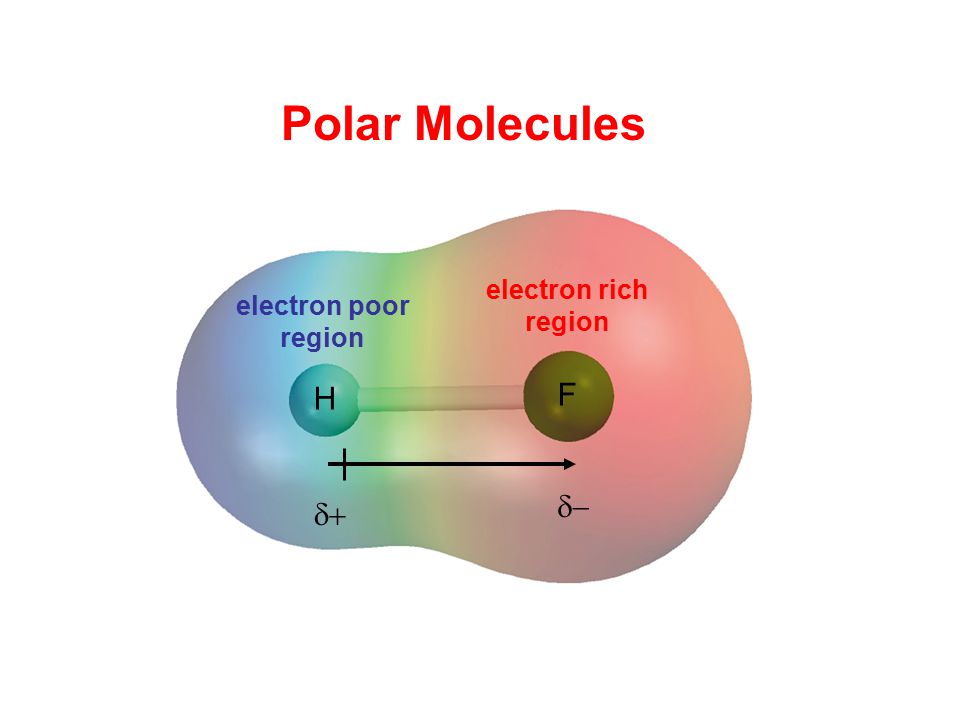
Hydrogen fluoride is a dipole.
Hf shape and polarity. Polarity of hf is nonpolar and it s molecular shape is linear. The small size and high electronegativity of fluorine is responsible for high polarity in hf molecules this high polarity is. Therefore the molecular polarity is the vector sum of the individual bond dipoles.
This leads to the development of a partial negative charge on the f atom and a partial positive charge on the h atom leading to the generation of a dipole and hence polarity. The hydrogen fluoride hf molecule is polar by virtue of polar covalent bonds. To determine the polarity of a covalent bond using numerical means find the difference between the electronegativity of the atoms.
Figure pageindex 2 a dipole is any molecule with a positive end and a negative end resulting from unequal distribution of electron density throughout the molecule. If molecular shape is symmatrical then its non polar but if it is non symmatrical then its polar. Each bond s dipole moment can be treated as a vector quantity having a magnitude and direction.
This results in a net dipole moment in a molecule. Ccl 2 f 2 d. How does molecular shape affect polarity.
Cf 2 h 2 e. Ni 3 trigonal pyramidal polar. We start with the lewis structure and then use vsepr to determine the shape of the.
Polarity of hf is nonpolar and it s molecular shape is linear. The polarization of a molecule greatly depends on the shape of the molecule. If the result is between 0 4 and 1 7 then generally the bond is polar covalent.
Hydrogen fluoride hf is a compound that is primarily polar. A diatomic molecule like hf mentioned above has no issue of. N 2 o i.
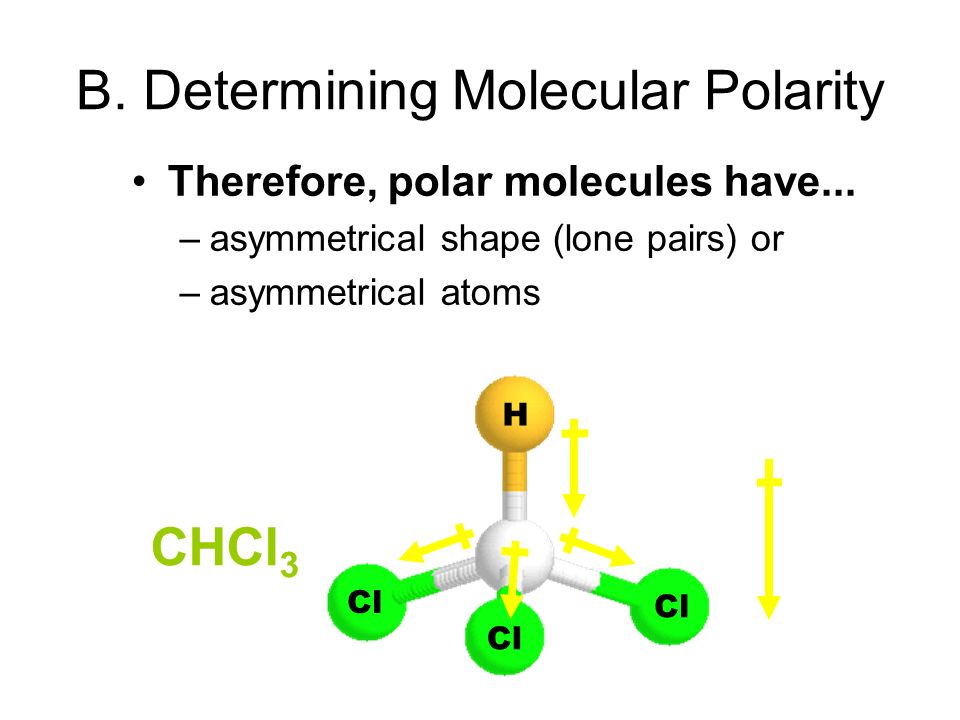
H 2 o m. The overall polarity of molecules with more than one bond is determined from both the polarity of the individual bonds and the shape of the molecule. For molecules with more than two atoms the molecular geometry must also be taken into account when determining if the molecule is polar or.
Hydrogen fluoride is a chemical compound with the chemical formula h f this colorless gas or liquid is the principal industrial source of fluorine often as an aqueous solution called hydrofluoric acid it is an important feedstock in the preparation of many important compounds including pharmaceuticals and polymers e g. The highly negative f in the hf molecule gets a slight negative charge while the h atom becomes slightly positive. This is due to the high electronegativity of the fluorine that pulls the shared electron pair between h and f more towards its side.
Hf linear polar s. Draw lewis structures name shapes and indicate polar or non polar for the following molecules. In the covalent bond electrons are displaced.



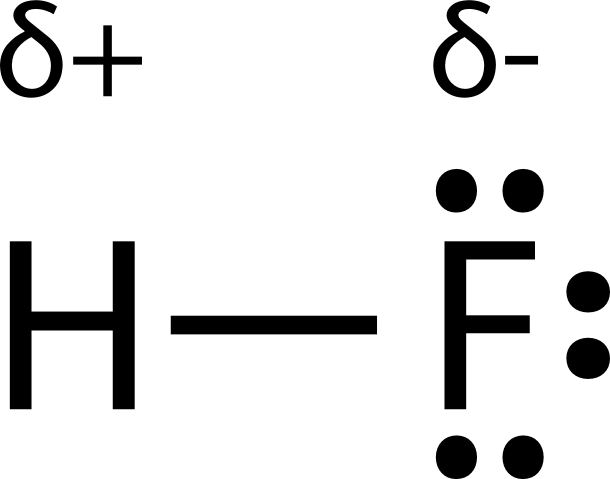






.PNG)